Development of a Simple and Effective Water Disinfection System Using an Electrochemical Mixed Oxidants-Generating System to Remove Waterborne Pathogens in Developing Countries
Article information
Abstract
Despite tremendous efforts from various international agencies such as World Health Organization (WHO), waterborne diseases are a still major cause of morbidity and mortality in the world – with more than 1 billion incidences and more than 2 million deaths per year. As an effort to mitigate this global burden, we developed a water disinfection system using electrochemical mixed oxidants-generating system. This system is simple, inexpensive, and easy to use. In this study, we determined the effectiveness of the new water disinfection system against waterborne pathogens using Escherichia coli CN13, bacteriophage MS2 and Bacillus subtilis spores as bacterial, viral, and protozoa indicator organisms, respectively. The results of this study showed that the new water disinfection system is very effective against E. coli CN13 (~3 log10 inactivation within 3 minutes with 0.3 mg/L of free chlorine) and coliphage MS2 (> 4 log10 inactivation within 3 minutes with 0.5 mg/L of free chlorine) Although the inactivation of B. subtilis spores by the new disinfection system was somewhat slow (~0.5 log10 inactivation in 60 minutes with 10 mg/L of free chlorine), the result is similar to previous studies with free chlorine. Overall, the results of this study indicate that the new water disinfection system using an electrochemical mixed oxidants-generating system is easy to use, convenient to carry, and also very effective against most waterborne pathogens, so it could be a sustainable solution for providing safe drinking water to the people in developing countries.
Introduction
Waterborne diseases are a major cause of morbidity and mortality in developing countries. It is difficult to estimate the precise number of incidence, but annual incidence of waterborne infectious diseases is estimated to be more than 1 billion with more than 2 million deaths (Payment and Riley, 2002; World Development Report, 1993; Burnet et al., 2014; Su et al., 2014; Ford and Hammer, 2018). More importantly, most (~90%) of the deaths happen among the children under 5 years old (Payment and Riley, 2002). In order to mitigate this global burden, numerous water treatment technologies have been developed and one of the promising technologies is an on-site electrochemical mixed oxidants-generating system. An electrochemical mixed oxidants-generating system can easily generate various oxidants (chlorine as well as ozone, chlorine dioxide, and hydrogen peroxide) simply by electrolysis of sodium chloride (NaCl) (Kim et al., 2018; Choi and Lee, 2019; Choi et al., 2022). This system is inexpensive, easy to use, and most importantly, can be produced on-site so that it could provide a sustainable solution to the waterborne diseases in developing countries.
In a previous study, we developed an on-site electrochemical mixed oxidants-generating system combined with solar energy (Figure 1(a) and (c)) and demonstrated that it could produce high concentration of chlorine (Choi et al., 2013). Later, we were able to downsize the original electrochemical mixed oxidants-generating system up to 1 L and made it convenient to carry (portable) (Figure 1(b)), and found out that this system can generate enough chlorine in a short period of time to disinfect a relatively large amount of water (Kim et al., 2015).
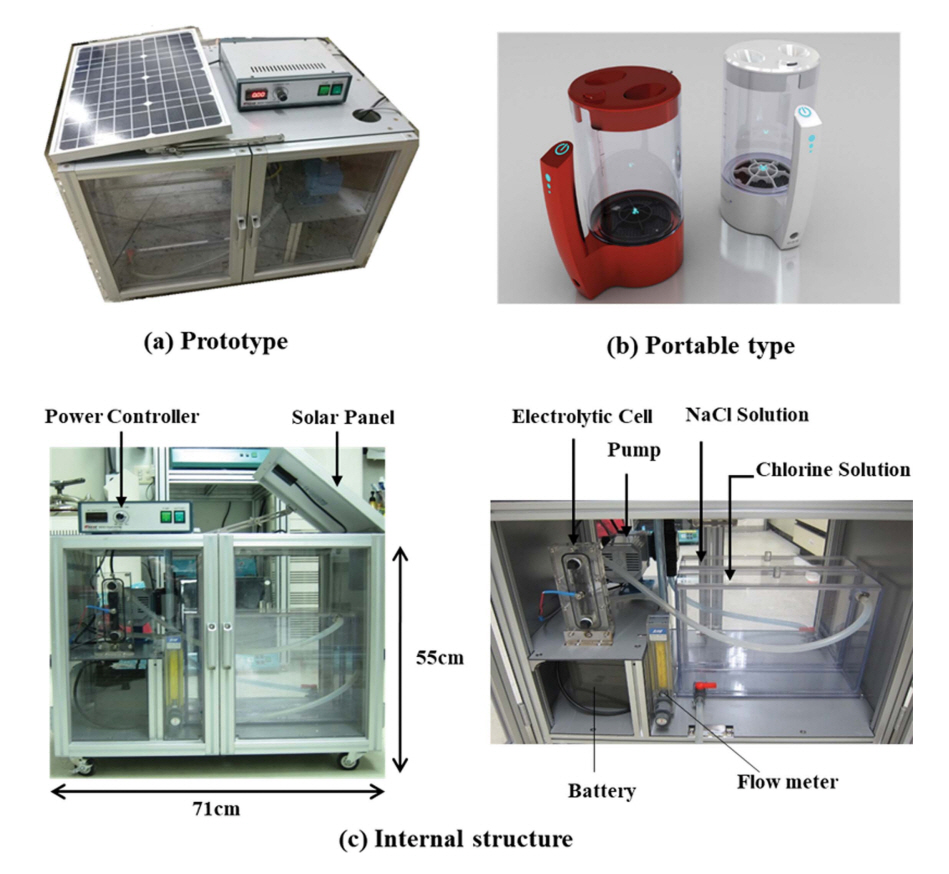
Water disinfection systems using an electrochemical mixed oxidants-generating system (a) a prototype, (b) a portable type, (c) internal structure of the prototype.
In this study, we determined the effectiveness of the new water disinfection system using an electrochemical mixed oxidants-generating system in terms of removal of various waterborne pathogens. In fact, there are hundreds of pathogens which can be transmitted through water in developing countries. Among them, we selected three representative organisms that have been used as indicators of disinfection efficacy in many previous studies (Casteel et al., 2000; Venczel et al., 2004; Son et al., 2005) - Escherichia coli CN13, bacteriophage MS2 and Bacillus subtilis spores as bacterial, viral, and protozoa indicator organisms, respectively.
Materials and Methods
1. Electrochemical mixed oxidants-generating systems
The original water disinfection systems using an electrochemical mixed oxidants-generating system (Prototype, Figure 1(a) and (c)) was described in a previous study (Choi et al., 2013). Briefly, the prototype consists of anode, cathode, 2 L reactor, a solar panel and a recharging battery. Both anode and cathode are DSA (dimensionally stable Anode) where Ru0.4Ir0.6O2 is coated on the Ti mesh substrate. Chlorine (Cl2) is generated on the anode with oxidation of chloride ion (Cl-) at 1.36 V (vs. NHE), and generated chlorine is dissolved in water as hypochlorous acid (HOCl). Meanwhile, hydrogen is generated on the cathode (Eq. (1)-(3)). On the other hand, a modified water disinfection system (Portable type. Figure 1(b)) is composed of anode, cathode, 1 L reactor, and power supply. Anode is DSA where Ru0.4Ir0.6O2 is coated on the Ti mesh substrate, and cathode is Pt coated Ti mesh. Generation of chlorine by portable system is similar to that of prototype shown in Eq. (1)-(3).
2. Microorganisms
The images of microorganisms used in this study were shown in Figure 2. E. coli CN13 (which has been used for an indicator organism for waterborne pathogenic bacteria (Casteel et al., 2000; Venczel et al., 2004) was acquired from available stocks of the Environmental Microbiology Laboratory at the University of North Carolina at Chapel Hill. For propagation, 10 μL of stock E. coli CN13 was inoculated into 30 mL of sterile Tryptic Soy Broth (TSB) and incubated at 37°C overnight with shaking. The overnight culture was centrifuged for 10 min at 1000 ×g at 20°C and the pellet was resuspended in PBS (pH 7.4). F-specific coliphage MS2 (ATCC #: 15597-B1) (which has been used for an indicator organism for waterborne pathogenic viruses (Casteel et al., 2000; Venczel et al., 2004) was grown in E. coli C3000 (ATCC #: 15597) by the double agar layer technique (Berman et al., 1988). The top agar layer exhibiting confluent lysis of the host cells was harvested by scraping into a small amount of PBS, and coliphage MS2 was extracted by homogenizing in an equal volume of chloroform. The supernatant was recovered following low speed (4,000 ×g) centrifugation for 30 minutes at 4°C. B. subtilis var. niger (ATCC strain S/O 373040) spores (which has been used for an indicator organism for waterborne pathogenic protozoa (Son et al., 2005). Although B. subtilus is not a protozoa, it has been used for an indicator organism for waterborne pathogenic protozoa due to its high resistance to water and wastewater disinfection) were produced in AK Agar #2 (Becton Dickinson, Cockeysville, MD). After sporulation, the suspension was heated to 80°C for 30 min to ensure destruction of vegetative cells. After eliminating vegetative cells, the titer of B. subtilis spores was determined by plating dextrose-tryptone (DT) agar.

Microorganisms used in this study (a) Bacteriophage MS2 (Dika et al., 2011) (b) Escherichia coli CN13 (Ciociola et al., 2018) (c) Bacillus subtilis (with endospores) (Erlendsson et al., 2002).
3. Free chlorine solutions and measurement of free chlorine residuals
Three different sources of free chlorine were used in this study - the household bleach (hereafter “Clorox”), the prototype electrochemical mixed oxidants-generating system (hereafter “prototype”), and the portable type electrochemical mixed oxidants-generating system (hereafter “portable”). Household bleach (5.25% sodium hypochlorite, Clorox Company, Oakland, CA) was used a control in this study for comparison with electrochemical mixed oxidants-generating systems. Clorox was diluted in CDF water to prepare a 100 mg/L stock solution. Working solutions of free chlorine at different concentrations were prepared in a 0.01 M phosphate buffer solution at pH 7 on the days of disinfection experiments. The prototype electrochemical mixed oxidants-generating system was operated with 35 g/L of NaCl for 1 hour with flow rate of 30 mL/min and 1 A of constant current. A portable electrochemical mixed oxidants generating system was operated with 10 g/L of NaCl for 5-10 min with 3 A of constant current. The concentration of the produced chlorine (chlorine residuals) were measured according to the N, N diethyl-p-phenylenediamine (DPD) colorimetric method (American Public Health Association, 1985).
4. Experimental protocol
Glassware for experiments and for reagents was prepared as previously described (Shin and Sobsey, 2008). Disinfection experiments were done in a bench scale, batch system using CDF glass test tubes placed in a water bath set at 5°C (A worst-case scenario in water treatment processes, In extreme cases such as winter time in certain areas, water temperature is sometimes near 0°C and disinfection at this low temperature is generally not as effective as at normal water temperature) as previously described (Sobsey et al., 1988).
5. Infectivity assays
E. coli CN13 and B. subtilis spores were assayed on Tryptic Soy Agar (TSA) and DT agar, respectively, by spread plating technique. Coliphage MS2 was assayed by the double agar layer plaque technique on host E. coli C3000 as previously described (Adams, 1959).
6. Data presentation
Disinfection data was summarized and presented the same as previously described (Shin and Sobsey, 2008). Statistical analysis on the kinetics of microbial inactivation was performed by using SAS (SAS Institute Inc., Cary, NC) and EXCEL (Microsoft, Redmond, WA) as previously described (Bae and Shin, 2016).
Results
1. Inactivation of E. coli CN13
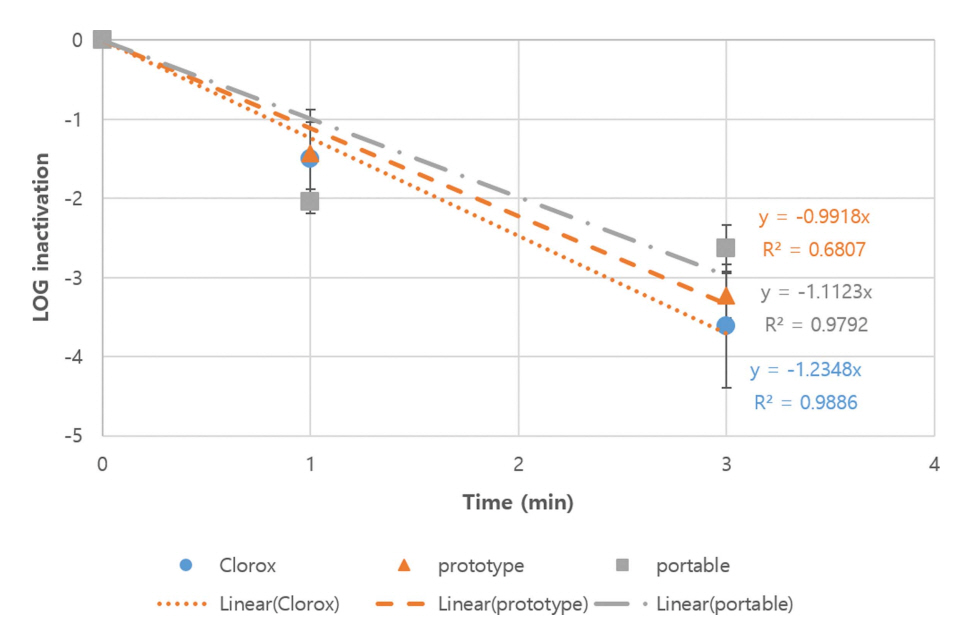
Figure 3 shows the inactivation kinetics of E. coli CN13 (an indicator organism for waterborne pathogenic bacteria) by 0.3 mg/L dose of the three different sources of free chlorine (from Clorox, prototype electrochemical mixed oxidants-generating system, and portable type electrochemical mixed oxidantsgenerating system) in a buffered, demand-free water (PBS) at pH 7 and 5°C based on the results of four independent (each duplicate) experiments. The inactivation kinetics of E. coli CN13 by Clorox (control) in this study was similar to those in previous studies (Berman et al., 1988). Meanwhile, the inactivation of E. coli CN13 by the three different sources of free chlorine was statistically different (ANCOVA, p value = 0.0150). The inactivation by Clorox appears to be the most effective, prototype to be the next, and portable type to be the least effective. As mentioned earlier, electrochemical mixed oxidants-generating systems can produce not only free chlorine but also some other “mixed oxidants”. However, those other mixed oxidants have not been well characterized and their actual concentration and proportion may depend upon other factors such as cell design, anode materials, and purity and concentration of salts (Venczel et al., 2004). Nonetheless, the inactivation of E. coli CN13by all the three sources of free chlorine were very rapid (approximately first-order), and a ~3 log10 inactivation was achieved within 3 minutes.
2. Inactivation of bacteriophage MS2
Figure 4 shows the inactivation kinetics of bacteriophage MS2 (an indicator organism for waterborne pathogenic viruses) by 0.5 mg/L dose of the three different sources of free chlorine (from Clorox, prototype electrochemical mixed oxidants-generating system, and portable type electrochemical mixed oxidants-generating system) in buffered, demand-free water at pH 7 and 5°C based on the results of three independent (each duplicate) experiments. The inactivation kinetics of bacteriophage MS2 by Clorox (control) in this study was similar to those in previous studies (Shin and Sobsey, 2008; Sobsey et al., 1988). Again, the inactivation of bacteriophage MS2 by the three types of free chlorine was statistically different (ANCOVA, p value = 0.0004). The inactivation by the prototype appears to be the most effective, Clorox to be the next, and portable type to be the least effective. Nevertheless, the inactivation of bacteriophage MS2 by all the three sources of free chlorine were also very rapid (approximately first order), and a ~4 log10 inactivation was achieved within 1 minutes.
3. Inactivation of Bacillus subtilis spores
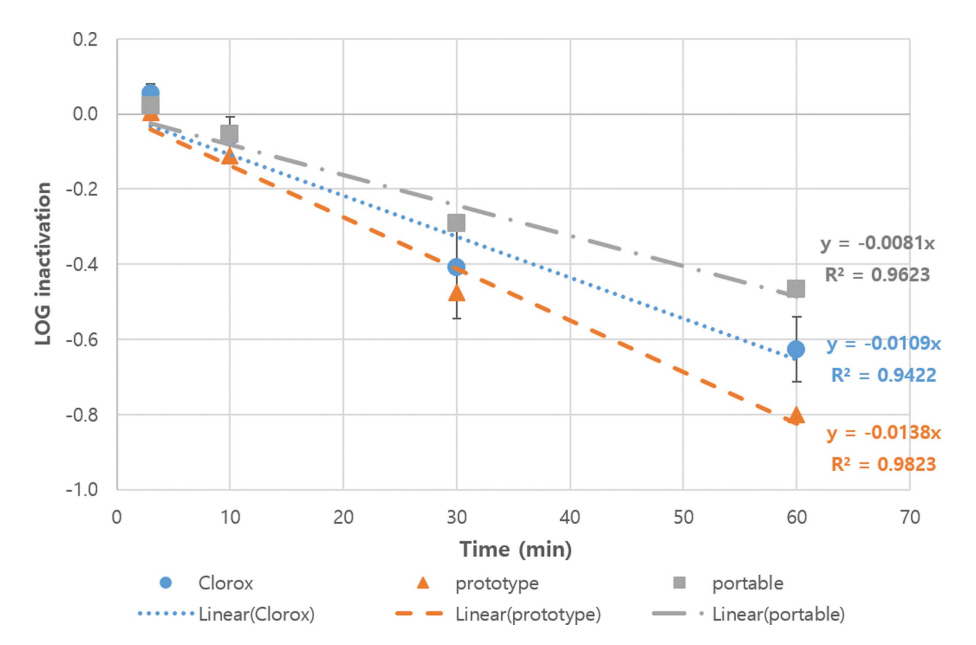
Figure 5 shows the inactivation kinetics of B. subtilis spores (an indicator organism for waterborne pathogenic protozoa) by 10 mg/L dose of the three types of free chlorine (from Clorox, prototype electrochemical mixed oxidants-generating system, and portable type electrochemical mixed oxidants-generating system) in buffered, demand-free water at pH 7 and 5°C based on the results of four independent (each duplicate) experiments. The inactivation kinetics of B. subtilis spores by Clorox (control) in this study was similar to those in a previous study (Son et al., 2005). Again, the inactivation of B. subtilis spores by the three different sources of free chlorine was statistically different (ANCOVA, p value < 0.001. The inactivation by the prototype appears to be the most effective, Clorox to be the next, and portable type to be the least effective. Compared to E. coli and bacteriophage MS2, however, the inactivation of B. subtilis spores by all the three sources of free chlorine were somewhat slow (approximately first-order), and ~1 log10 inactivation was achieved in 60 minutes.
Discussion
The results of this study showed that the electrochemical mixed oxidants generating systems - both prototype and portable type - were very effective against E. coli CN13 and bacteriophage MS2. About 3 log10 inactivation of E. coli CN13 was achieved within 3 minutes with 0.3 mg/L of free chlorine and ~4 log10 inactivation of bacteriophage MS2 was achieved within 3 minutes by 0.5 mg/L of free chlorine from the two. electrochemical mixed oxidants generating systems. Since E. coli CN13 and bacteriophage MS2 are one of the most widely used indicator organisms for waterborne pathogenic bacteria and viruses respectively (Casteel et al., 2000; Venczel et al., 2004), it is likely that the electrochemical mixed oxidants generating systems developed in this study can remove most of waterborne bacteria and viruses in developing countries. On the other hand, the results of this study showed that the two electrochemical mixed oxidants generating systems were not so effective against B. subtilis spores (an indicator organism for waterborne pathogenic protozoa). There was only ~1 log10 inactivation of B. subtilis spores in 60 minutes with 10 mg/L of free chlorine from the two systems. Compared to waterborne bacteria and viruses, however, waterborne protozoa are a lot more resistant to free chlorine (Sobsey, 1989). Therefore, it usually requires higher concentration of free chlorine with long contact time to achieve significant inactivation of waterborne protozoa Nonetheless, simple physico-chemical water treatment systems such as sand filtration ahead of mixed oxidants generating system should be able to achieve a significant removal of waterborne protozoa in developing countries.
Also, the results of this study indicated that there was some statistically significant difference among the three sources of free chlorine in terms of their effectiveness against various waterborne microorganisms. Although free chlorine is the primary oxidant generated in an electrochemical mixed oxidants generating system, there are some other oxidants such as ozone, hydrogen peroxide, and hydroxyl free radicals generated in certain conditions (Venczel et al., 2004) There are some studies that showed higher effectiveness of mixed oxidants than free chlorine possibly due to those additional oxidants (Venczel et al., 2004; Son et al., 2005). It should be mentioned however, that the concentration of oxidants output is proportional not only to the concentration of salt in the input, but also other factors such as voltage, temperature, current and electrolysis time. In order to increase the convenience, we reduced not only the size, but also the amount of salts, current, and electrolysis time in the portable type. Despite all these modifications, there was only slight decrease in terms of effectiveness against the microorganisms tested in the portable type.
Overall, the results of this study suggest that the water disinfection system generating mixed oxidants developed in this study can provide a cheap and effective disinfection against most waterborne pathogens in developing countries. Most importantly, the portable type developed in this study is easy to use, convenient to carry, and also very effective against most waterborne pathogens, so it could be a sustainable solution for providing safe drinking water to the people in developing countries.
Acknowledgements
This research is financially supported by Global Top Environmental Technology Development (E61600211-0689-0) funded by Korean Ministry of Environment.